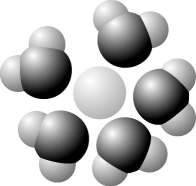
-Based on your knowledge of the polarity of water molecules, the solute molecule depicted here is most likely
A) positively charged.
B) negatively charged.
C) without charge.
D) hydrophobic.
E) nonpolar.
Correct Answer:
Verified
Q46: Assume that acid rain has lowered the
Q47: Equal volumes (5 mL)of vinegar from a
Q48: Identical heat lamps are arranged to shine
Q49: Carbon dioxide (CO₂)is readily soluble in water,
Q50: A beaker contains 100 mL of NaOH
Q52: How would acidification of seawater affect marine
Q53: If the cytoplasm of a cell is
Q54: If a solution has a pH of
Q55: Consider two solutions: solution X has a
Q56: ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents