Identical heat lamps are arranged to shine on identical containers of water and methanol (wood alcohol) , so that each liquid absorbs the same amount of energy minute by minute. The covalent bonds of methanol molecules are nonpolar, so there are no hydrogen bonds among methanol molecules. Which of the following graphs correctly describes what will happen to the temperature of the water and the methanol?
A) 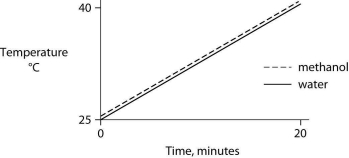
B) 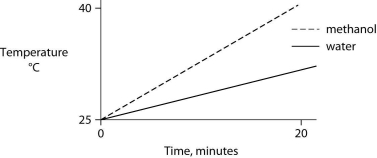
C) 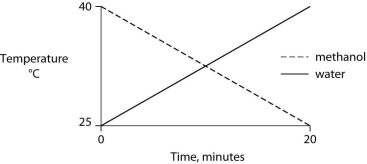
D) 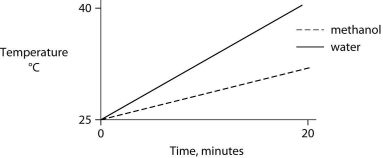
E) 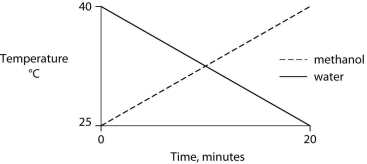
Correct Answer:
Verified
Q43: Increased atmospheric CO₂ concentrations might have what
Q44: A small birthday candle is weighed, then
Q45: Which of these molecules would be soluble
Q46: Assume that acid rain has lowered the
Q47: Equal volumes (5 mL)of vinegar from a
Q49: Carbon dioxide (CO₂)is readily soluble in water,
Q50: A beaker contains 100 mL of NaOH
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents