For the hydrolysis of ATP to ADP + 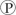 ᵢ, the free energy change is -7.3 kcal/mol under standard conditions (1 M concentration of both reactants and products) . In the cellular environment, however, the free energy change is about -13 kcal/mol. What can we conclude about the free energy change for the formation of ATP from ADP and
ᵢ, the free energy change is -7.3 kcal/mol under standard conditions (1 M concentration of both reactants and products) . In the cellular environment, however, the free energy change is about -13 kcal/mol. What can we conclude about the free energy change for the formation of ATP from ADP and 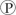 ᵢ under cellular conditions?
ᵢ under cellular conditions?
A) It is +7.3 kcal/mol.
B) It is less than +7.3 kcal/mol.
C) It is about +13 kcal/mol.
D) It is greater than +13 kcal/mol.
E) The information given is insufficient to deduce the free energy change.
Correct Answer:
Verified
Q32: When 10,000 molecules of ATP are hydrolyzed
Q33: Which of the following best describes enthalpy
Q34: During a laboratory experiment, you discover that
Q35: Increasing the substrate concentration in an enzymatic
Q36: When chemical, transport, or mechanical work is
Q38: When ATP releases some energy, it also
Q39: What is the difference (if any)between the
Q40: Which of the following statements is true
Q41: How might an amino acid change at
Q42: ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents