What angle does the orbital angular momentum make with the z axis of a hydrogen atom in the state n = 3, 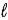 = 2,
= 2, 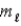 = −1?
= −1?
A) −66°
B) 66°
C) 24°
D) 114°
E) 73°
Correct Answer:
Verified
Q32: The probability density of a particle at
Q33: The magnitude of the spin angular momentum
Q34: Forbidden transitions and selection rules suggest that
A)
Q35: When electrons fill a subshell in which
Q36: For the following allowed transitions, which photon
Q38: The ground state configuration of chlorine (Z
Q39: In a completely filled atomic shell,
A) the
Q40: The Pauli Exclusion Principle states
A) no two
Q41: Which of the following, in which n
Q42: What is the difference in frequency for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents