Quantum physics agrees with the classical physics limit when
A) the total angular momentum is a small multiple of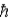 .
.
B) the total energy is a small multiple of the energy in the lowest quantized state.
C) the difference in energy between adjacent quantized levels becomes vanishingly small.
D) all electron spins are paired so that L = 0.
E) there is a vacancy in an inner level in the atom.
Correct Answer:
Verified
Q40: The Pauli Exclusion Principle states
A) no two
Q41: Which of the following, in which n
Q42: What is the difference in frequency for
Q43: A hydrogen atom emits a photon of
Q44: A headwaiter at a restaurant decides to
Q46: Adam and Eve are contemplating the beauty
Q47: The energy difference between the upper and
Q48: What is the difference in wavelength for
Q49: Aline says that the magnetic moment of
Q50: The number of electrons in the n
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents