A gas expands as shown in the graph. If the heat taken in during this process is 1.02 × 106 J and 1 atm = 1.01 × 105 N/m2, the change in internal energy of the gas (in J) is 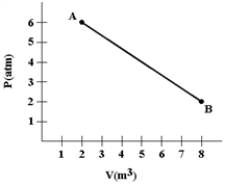
A) −2.42 × 106
B) −1.40 × 106
C) −1.02 × 106
D) 1.02 × 106
E) 1.40 × 106
Correct Answer:
Verified
Q26: In an isovolumetric process
A) the temperature remains
Q27: Which statement below regarding the First Law
Q28: One gram of water is heated from
Q29: Five moles of an ideal gas expands
Q30: How much heat, in joules, is required
Q32: If an object feels cold to the
Q33: Determine the work done by 5 moles
Q34: In an isobaric process
A) the volume remains
Q35: An 8 000-kg aluminum flagpole 100-m high
Q36: Two kilograms of water at 100°C is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents