Nuclear reactions: In the nuclear reaction
n + 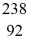 U → X + 2e-
U → X + 2e-
n is a neutron and e- is an electron, and the neutrinos have not been shown. Determine the atomic mass and atomic number of the missing nuclear product X, and write X in the standard form. It is NOT necessary to identify which atom X is.
Correct Answer:
Verified
Q56: Radioactive decay: A hospital patient has been
Q57: Radioactivity: The stability of Q58: Radioactive decay: The unstable isotope 234Th decays Q59: Radioactivity: Scandium, Q60: Radioactive dating: The radioactivity due to carbon-14 Q62: Radioactive dating: An ancient rock is found Q63: Nuclear fission: How much energy is released Q64: Radioactive dating: Living matter has 1.3 × Q65: Nuclear reactions: For the missing product X Q66: Nuclear fission: In the fission reaction ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents