Radioactivity: The stability of 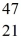 Sc with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known:
Sc with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known: 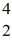 He: 4.002603 u
He: 4.002603 u 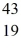 K: 42.960717 u
K: 42.960717 u 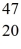 Ca: 46.954543 u
Ca: 46.954543 u 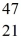 Sc 46.952409 u
Sc 46.952409 u 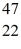 Ti: 46.951764 u The
Ti: 46.951764 u The 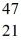 Sc nuclide is
Sc nuclide is
A) not subject to alpha, β+, or β- decay.
B) subject to alpha decay only.
C) subject to β+ decay only.
D) subject to β- decay only.
E) subject to β+ or β- decay, but not to alpha decay.
Correct Answer:
Verified
Q52: Radioactive decay: A certain substance has a
Q53: Radioactive decay: What mass of 14C (having
Q54: Radioactive decay: An isotope of Tc having
Q55: Radioactive dating: Carbon-14 has a half-life of
Q56: Radioactive decay: A hospital patient has been
Q58: Radioactive decay: The unstable isotope 234Th decays
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents