Ideal gas law: A sealed 26- 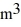 tank is filled with 2000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 460 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The final pressure of the gas is closest to
tank is filled with 2000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 460 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The final pressure of the gas is closest to
A) 0.29 MPa.
B) 0.31 MPa.
C) 0.33 MPa.
D) 0.34 MPa.
E) 0.36 MPa.
Correct Answer:
Verified
Q79: Radiation: The filament in a light bulb
Q80: Conduction of heat: A cylindrical bar that
Q81: Ideal gas law: A hot air balloon
Q82: Molecular speeds: A 5.0-liter gas tank holds
Q83: Ideal gas law: A 25-L container holds
Q85: Ideal gas law: A sealed container holds
Q86: Ideal gas law: How many moles of
Q87: Molecular speeds: An ideal gas is kept
Q88: Ideal gas law: A cold trap is
Q89: Ideal gas law: A bag of potato
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents