Ideal gas law: A cold trap is set up to cause molecules to linger near the suction in a vacuum system. If the cold trap has an effective volume of 0.200 L and is maintained at 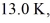 how many molecules are in it at 10.0 Pa of pressure? (Avogadro's number is 6.022 × 1023 molecules/mol, and the universal gas constant is 8.314 J/mol ∙ K. Assume the behavior of an ideal gas.)
how many molecules are in it at 10.0 Pa of pressure? (Avogadro's number is 6.022 × 1023 molecules/mol, and the universal gas constant is 8.314 J/mol ∙ K. Assume the behavior of an ideal gas.)
A) 1.11 × 1019 molecules
B) 1.10 × 1022 molecules
C) 7.71 × 1020 molecules
D) 7.71 × 1023 molecules
Correct Answer:
Verified
Q83: Ideal gas law: A 25-L container holds
Q84: Ideal gas law: A sealed 26-
Q85: Ideal gas law: A sealed container holds
Q86: Ideal gas law: How many moles of
Q87: Molecular speeds: An ideal gas is kept
Q89: Ideal gas law: A bag of potato
Q90: Ideal gas law: The interior of a
Q91: Ideal gas law: An ideal gas is
Q92: Molecular speeds: The root-mean-square speed (thermal speed)
Q93: Ideal gas law: 3.00 moles of an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents