For the exothermic reaction 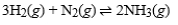 making which of the following changes will ensure that the reaction will not shift either to the left or to the right?
making which of the following changes will ensure that the reaction will not shift either to the left or to the right?
A) adding H2 and N2 and removing NH3
B) adding H2 and lowering the temperature
C) adding H2 and raising the temperature
D) adding H2 and decreasing the volume of the beaker
Correct Answer:
Verified
Q78: For the reaction Q79: Which of the following will change the Q80: For the reaction Q81: What is the effect of adding CH3COOC2H5(g)to Q82: Which of the following is not true Q84: Consider the following energy diagram for a Q85: A particular reaction has an equilibrium constant Q86: Consider the following energy diagram for a Q87: Consider the following energy diagram for a Q88: When considering the effect of a catalyst![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents