Consider the following energy diagram for a reaction. 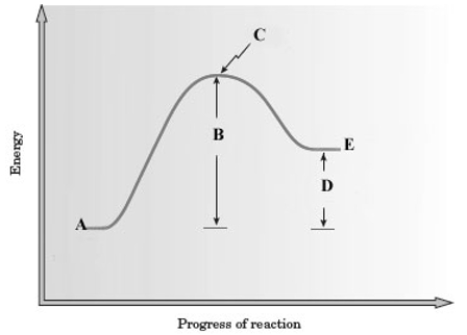 Which letter represents the net energy change of the reaction?
Which letter represents the net energy change of the reaction?
A) A
B) B
C) C
D) D
E) E
Correct Answer:
Verified
Q81: What is the effect of adding CH3COOC2H5(g)to
Q82: Which of the following is not true
Q83: For the exothermic reaction Q84: Consider the following energy diagram for a Q85: A particular reaction has an equilibrium constant Q87: Consider the following energy diagram for a Q88: When considering the effect of a catalyst Q89: How does a decrease in temperature affect Q90: To which of the following systems does Q91: How does a decrease in temperature affect![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents