For the exothermic reaction 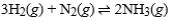 making which of the following changes will ensure that the reaction will not shift either to the left or to the right?
making which of the following changes will ensure that the reaction will not shift either to the left or to the right?
A) raising the temperature and adding NH3
B) lowering the temperature and removing NH3
C) raising the temperature and adding NH3 and lowering the temperature and removing NH3
D) none of these
Correct Answer:
Verified
Q93: If the endothermic reaction Q94: What is the effect of adding CH3COOH(g) Q95: If the exothermic reaction Q96: For the exothermic reaction Q97: Which of the following is the equilibrium Q99: Consider the following energy diagram for a Q100: Consider the following energy diagram for a Q101: In the reaction of HCl with water: Q102: Consider the following graph. Q103: Consider the following graph. Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()
![]()
![]()

