Which is the stronger base if the equilibrium lies to the right? (Sec. 2.4, HARD) 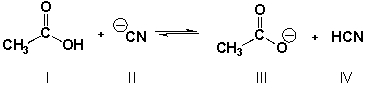
A) I
B) II
C) III
D) IV
Correct Answer:
Verified
Q3: Identify the conjugate acids in the following
Q4: Identify the Arrhenius acids:
I. HCl
II. NaOH
Q5: List the bonds in order of decreasing
Q6: Which equilibria have equilibrium constants greater than
Q7: Which are acid-base reactions according to Brønsted-Lowry
Q9: Which ion is the strongest base?
Q10: Identify the conjugate bases in the following
Q11: Identify the Arrhenius bases:
I. NH3
II. NaOH
Q12: Which of these has the lowest numerical
Q13: Identify the Brønsted-Lowry acids in the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents