Which Lewis structures are correct?
HINT: Perform a total valence count and check formal charges.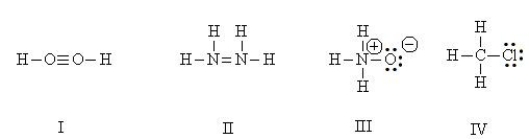
A) I, II
B) II, IV
C) III, IV
D) I, III
Correct Answer:
Verified
Q14: Nitrogen has a negative formal charge in
Q15: Arrange the bonds in increasing order of
Q16: Using the VSEPR model, predict which atoms
Q17: Which atom is described by the electron
Q18: Which atom is described by the Lewis
Q20: According to VSEPR model, what is your
Q21: Which compounds are classified correctly?
HINT: Assume that
Q22: _ is the number of valence electrons
Q23: What is the correct structure for the
Q24: Using the VSEPR model, predict which molecules
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents