Using the VSEPR model, predict which molecules have bond angles of about 120°.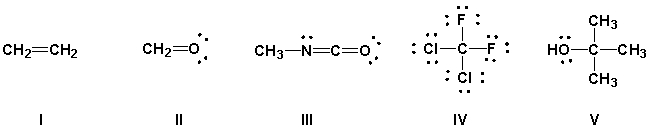
A) I, II, IV
B) I, II, III
C) III, IV, V
D) II, III, IV
Correct Answer:
Verified
Q19: Which Lewis structures are correct?
HINT: Perform a
Q20: According to VSEPR model, what is your
Q21: Which compounds are classified correctly?
HINT: Assume that
Q22: _ is the number of valence electrons
Q23: What is the correct structure for the
Q25: Outer shell electrons are called _ electrons.
Q26: The carbon has the correct orbital hybridization
Q27: Which of the three molecules aspirin, paracetamol
Q28: What is the formal charge of indicated
Q29: Which compounds contain both covalent and ionic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents