What is ΔG° for the following reaction? (F = 96,500 C • mol -1) I2(s) + 2Br-(aq) → 2I-(aq) + Br2(l)
Given: 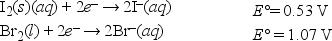
A) +104 kJ/mol
B) -104 kJ/mol
C) +309 kJ/mol
D) +52 kJ/mol
E) -52 kJ/mol
Correct Answer:
Verified
Q55: What is ΔG° at 200°C for the
Q56: Based on the data presented below, which
Q57: What is ΔG° at 298 K for
Q58: Based on the data presented below, which
Q59: Which metals may be oxidized by H+
Q61: Which, if any, of the following metals
Q62: Which metal is not capable of acting
Q63: What mass of nickel may be electroplated
Q64: A metal object is to be gold-plated
Q65: Which is the half-reaction at the anode
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents