Which, if any, of the following metals would be capable of acting as a sacrificial anode when used with iron pipe? 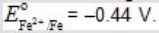
A) 
B) 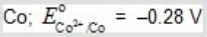
C) 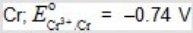
D) 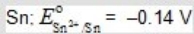
E) 
Correct Answer:
Verified
Q56: Based on the data presented below, which
Q57: What is ΔG° at 298 K for
Q58: Based on the data presented below, which
Q59: Which metals may be oxidized by H+
Q60: What is ΔG° for the following reaction?
Q62: Which metal is not capable of acting
Q63: What mass of nickel may be electroplated
Q64: A metal object is to be gold-plated
Q65: Which is the half-reaction at the anode
Q66: A voltaic cell consists of a Hg/Hg22+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents