What is ΔS° for the reaction SO3(g) + H2O(l) → H2SO4(aq) ? 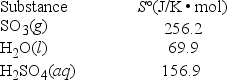
A) 169.2 J/K • mol
B) 343.2 J/K • mol
C) -169.2 J/K • mol
D) -29.4 J/K • mol
E) 29.4 J/K • mol
Correct Answer:
Verified
Q23: What is ΔS° for the reaction SO2(s)
Q24: What is ΔS° at 298 K for
Q25: What is ΔS° for the combustion of
Q26: What term is given to the fact
Q27: What is ΔS° for the following reaction?
Q29: Which is the thermodynamic condition for a
Q30: Which is true for a system at
Q31: Which is a statement of the third
Q32: What is ΔS° for the following reaction?
Q33: Which reaction has the largest ΔS°?
A) 2N2H4(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents