What is ΔS° for the following reaction? SiCl4(g) + 2Mg(s) → 2MgCl2(s) + Si(s) 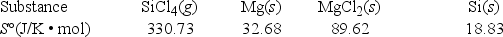
A) -254.96 J/K • mol
B) -198.02 J/K • mol
C) +198.02 J/K • mol
D) +254.96 J/K • mol
E) +471.86 J/K • mol
Correct Answer:
Verified
Q22: Which is necessary for a process to
Q23: What is ΔS° for the reaction SO2(s)
Q24: What is ΔS° at 298 K for
Q25: What is ΔS° for the combustion of
Q26: What term is given to the fact
Q28: What is ΔS° for the reaction SO3(g)
Q29: Which is the thermodynamic condition for a
Q30: Which is true for a system at
Q31: Which is a statement of the third
Q32: What is ΔS° for the following reaction?
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents