Suppose 50.0 mL of a 0.100 M solution of the weak acid HA is titrated with a 0.100 M solution of NaOH. Which diagram best represents the equilibrium composition of the solution once 25.0 mL of titrant have been added? (Solvent water molecules are omitted for clarity.)
A) 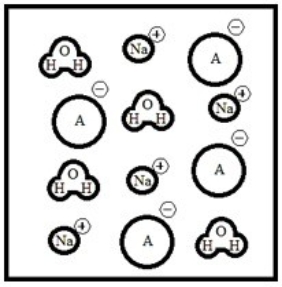
B) 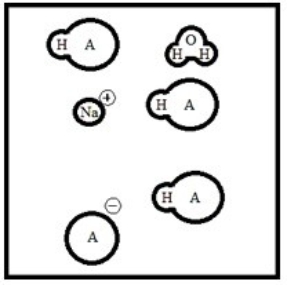
C) 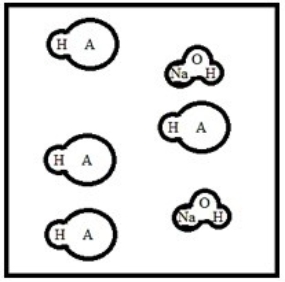
D) 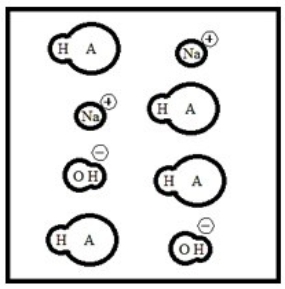
E) 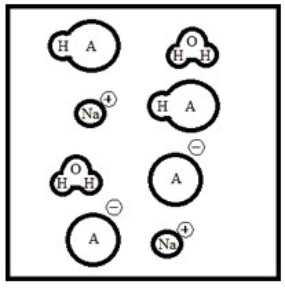
Correct Answer:
Verified
Q77: Calculate the minimum concentration of Mg2+ that
Q79: The solubility of lead(II) chloride is 0.45
Q80: Does a precipitate of magnesium fluoride form
Q81: Suppose 3.0 ×10-3 mol of NaOH are
Q83: Suppose 50.0 mL of a 0.100 M
Q84: Which is more soluble in an acidic
Q85: Below is a representation of the sparingly
Q86: Which is more soluble in a basic
Q87: Which is more soluble in an acidic
Q94: Calculate the solubility of zinc hydroxide, Zn(OH)2,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents