What is KP at 298 K for the following reaction? (R = 8.314 J/K • mol) SO2(g) + NO2(g) → SO3(g) + NO(g) 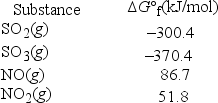
A) 6.99 × 10-7
B) 5.71 × 10-8
C) 14.2
D) 475
E) 1.42 × 106
Correct Answer:
Verified
Q43: The standard free energy of formation of
Q44: For the reaction H2(g) + Br2(g)
Q45: The equilibrium constant KP at 427°C for
Q46: A sample of solid naphthalene is introduced
Q47: In the gas phase, methyl isonitrile (CH3NC)
Q49: At 1500°C the equilibrium constant for the
Q50: Which is correct?
A) If ΔG < 0,
Q51: Which represents the correct relationship between the
Q52: Find the temperature at which KP =
Q53: At what temperature is KP = 4.00
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents