At what temperature is KP = 4.00 for the reaction N2O4(g)  2NO2(g) ?
2NO2(g) ? 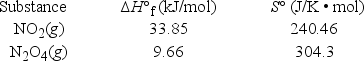 (R = 8.314 J/K • mol)
(R = 8.314 J/K • mol)
A) 197°C
B) 56°C
C) 36°C
D) 79°C
E) 476°C
Correct Answer:
Verified
Q48: What is KP at 298 K for
Q49: At 1500°C the equilibrium constant for the
Q50: Which is correct?
A) If ΔG < 0,
Q51: Which represents the correct relationship between the
Q52: Find the temperature at which KP =
Q54: Nitrosyl chloride (NOCl) decomposes at elevated temperatures
Q55: Hydrogen peroxide (H2O2) decomposes according to the
Q56: For the reaction PCl3(g) + Cl2(g)
Q57: The equilibrium constant for the reaction AgBr(s)
Q58: The solubility product constant, Ksp, at 25°C
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents