Given the following energy profiles, which of the following reactions is endothermic? 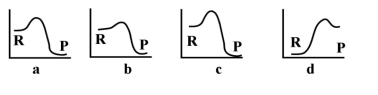 R= reactants P= products
R= reactants P= products
A) a
B) b
C) c
D) d
E) none of the above
Correct Answer:
Verified
Q24: How much energy, in kilojoules, is released
Q27: Use the bond energies below to determine
Q32: How many bonds between nitrogen and hydrogen
Q34: The reactants shown schematically below represent iron
Q69: What is a reaction rate?
A)It is the
Q98: What is an endothermic reaction?
A)It is a
Q101: Which is higher in an endothermic reaction:
Q110: Are the chemical reactions that take place
Q114: Which of the following reaction energies is
Q120: ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents