How much energy, in kilojoules, is released or absorbed from the reaction of one mole of nitrogen, N2, with three moles of molecular hydrogen, H₂, to form two moles of ammonia, NH₃? H-N (bond energy: 389 kJ/mol)
H-H (bond energy: 436 kJ/mol)
N 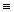 N (bond energy: 946 kJ/mol)
N (bond energy: 946 kJ/mol)
N 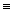 N + H-H + H-H → NH₃ + NH₃
N + H-H + H-H → NH₃ + NH₃
A) +899 kJ/mol absorbed
B) -993 kJ/mol released
C) +80 kJ/mol absorbed
D) -80 kj/mol released
Correct Answer:
Verified
Q19: A friend argues that if mass were
Q20: For the following balanced reaction, which of
Q22: How many moles of H₂ bonds are
Q27: Use the bond energies below to determine
Q29: Given the following energy profiles, which of
Q101: Which is higher in an endothermic reaction:
Q104: Bond energies increase in going from C-N
Q110: Are the chemical reactions that take place
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents