Dipole-induced dipole forces of attraction exist between water and gasoline, and yet these two substances do not mix because water has such a strong attraction for itself. Which of the following compounds might best help to make these two substances mix into a single liquid phase? 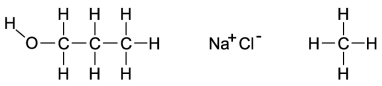
A) the molecule on the far left because the O-H bond is polar and the carbon and hydrogen bonds are nonpolar
B) the molecule in the middle because when the salts mix into the water, it will help separate the water and decrease the attraction for itself
C) The molecule on the right will form attractions with the polar ends of the water, allowing the gasoline a chance to mix with the water.
D) All of these molecules would be equally effective at increasing the mixing of gasoline and water.
Correct Answer:
Verified
Q13: A combination of two or more substances
Q17: What is the difference between a compound
Q137: Why are ion-dipole attractions stronger than dipole-dipole
Q142: Why is the surface area of a
Q144: How are oxygen molecules attracted to water
Q144: Why are the melting temperatures of most
Q151: The following image represents which kind of
Q153: List the following compounds in order of
Q155: Consider the boiling points of the following
Q160: The boiling point of 1,4-butanediol is 230°C.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents