Consider the boiling points of the following compounds and their solubilities in room-temperature water. Why does the solubilities in water go down as the boiling points of these alcohols go up. 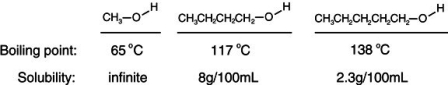
A) Larger molecules are less attracted to one another by induced dipole-induced dipole as well as by dipole-dipole and dipole-induced dipole attractions.
B) As the boiling increases, it is more difficult to keep the alcohol from evaporating out of solution.
C) As the boiling point increases, the size of the alcohol molecules decreases.
D) Larger molecules are more attracted to one another by induced dipole-induced dipole as well as by dipole-dipole and dipole-induced dipole attractions.
Correct Answer:
Verified
Q13: A combination of two or more substances
Q137: Why are ion-dipole attractions stronger than dipole-dipole
Q142: Why is the surface area of a
Q144: How are oxygen molecules attracted to water
Q144: Why are the melting temperatures of most
Q150: Why is calcium fluoride, CaF2, a high
Q151: The following image represents which kind of
Q153: List the following compounds in order of
Q156: Dipole-induced dipole forces of attraction exist between
Q160: The boiling point of 1,4-butanediol is 230°C.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents