Use the following information to answer the question below. 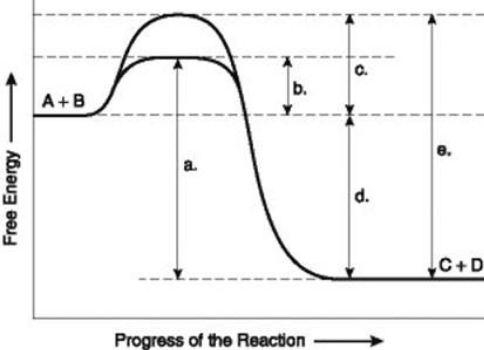 The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the following terms best describe the forward reaction in the figure?
The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the following terms best describe the forward reaction in the figure?
A) endergonic, ∆G > 0
B) exergonic, ∆G < 0
C) endergonic, ∆G < 0
D) exergonic, ∆G > 0
Correct Answer:
Verified
Q51: Use the following information to answer the
Q52: The 3-D structure of an enzyme composed
Q53: In addition to activating or inhibiting enzymes
Q54: A series of enzymes catalyze the reactions
Q55: A change of a single amino acid
Q57: Use the following information to answer the
Q58: Use the following information to answer the
Q59: Use the following information to answer the
Q60: An enzyme is composed of four identical
Q61: Choose the pair of terms that correctly
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents