Figure 9.1 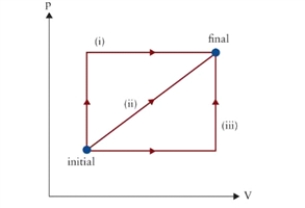 p-V diagram of several possible thermodynamic processes between initial and final states.
p-V diagram of several possible thermodynamic processes between initial and final states.
-A fixed mass of gas initially occupies a 2 L volume at atmospheric pressure. It undergoes an isobaric change to a volume of 5 L, followed by an isochoric change to a pressure of 2 atm. See path (iii) in Fig. 9.1. If the system is brought back to its initial state along the reverse of path (ii) , what is the net work done on the gas?
A) -456 J
B) -152 J
C) 152 J
D) 456 J
Correct Answer:
Verified
Q8: Figure 9.1 Q9: A monatomic ideal gas with an initial Q10: An expandable container holds 2.50 moles of Q11: A gas expands from an initial volume Q12: 2.0 moles of an ideal monatomic gas Q14: Which of the following statements best describes Q15: A gas expands from an initial volume Q16: In which one of these ways does Q17: A gas is heated at a constant Q18: How does the molar heat capacity of![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents