Figure 9.1 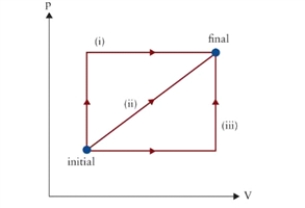 p-V diagram of several possible thermodynamic processes between initial and final states.
p-V diagram of several possible thermodynamic processes between initial and final states.
-In Fig. 9.1, by which closed path, starting and ending at "initial" and passing through "final," will the most work be done on the gas?
A) (iii) followed by reverse of (i)
B) (iii) followed by reverse of (ii)
C) (i) followed by reverse of (iii)
D) (ii) followed by reverse of (i)
Correct Answer:
Verified
Q3: An iron bullet of mass 100 g
Q4: In comparing adiabatic and isothermal compressions of
Q5: What happens during an isochoric process when
Q6: An athlete doing push-ups performs 700 kJ
Q7: An ideal monatomic gas expands at a
Q9: A monatomic ideal gas with an initial
Q10: An expandable container holds 2.50 moles of
Q11: A gas expands from an initial volume
Q12: 2.0 moles of an ideal monatomic gas
Q13: Figure 9.1 ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents