The temperature of a 350 mL sample of gas increases from 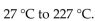
What is the final volume of the sample of gas, if the pressure and amount of gas in the container is kept constant?
A) 41.6 mL
B) 2940 mL
C) 583 mL
D) 110. mL
E) 210 .mL
Correct Answer:
Verified
Q27: A gas sample in a closed, expandable
Q28: According to Gay-Lussac's Law, the pressure of
Q29: When the combined gas law is rearranged
Q30: A sample of argon gas at
Q31: A balloon is filled with helium gas.
Q33: The temperature of a 500. mL sample
Q34: A sample of helium gas at
Q35: The volume of a sample of gas,
Q36: At 570. mm Hg and 
Q37: As the temperature of a gas increases,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents