At 570. mm Hg and 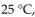 a gas sample has a volume of 2270 mL. What is the final pressure (in mmHg) at a volume of 1250 mL and a temperature of
a gas sample has a volume of 2270 mL. What is the final pressure (in mmHg) at a volume of 1250 mL and a temperature of 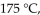 if the amount of gas does not change?
if the amount of gas does not change?
A) 690. mmHg
B) 1560 mmHg
C) 700 . mmHg
D) 470 . mmHg
E) 210. mmHg
Correct Answer:
Verified
Q31: A balloon is filled with helium gas.
Q32: The temperature of a 350 mL
Q33: The temperature of a 500. mL sample
Q34: A sample of helium gas at
Q35: The volume of a sample of gas,
Q37: As the temperature of a gas increases,
Q38: A diver exhales a bubble with a
Q39: What unit of temperature is used in
Q40: The boiling point of water at sea
Q41: According to Avogadro's law,
A)the volume of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents