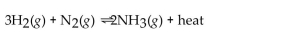 For the reaction at equilibrium, if the concentration of
For the reaction at equilibrium, if the concentration of 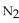 is increased, will the equilibrium shift in the direction i reactants, products, or stay the same?
is increased, will the equilibrium shift in the direction i reactants, products, or stay the same?
A) reactants
B) products
C) stay the same
Correct Answer:
Verified
Q36: Q37: For the following reaction, the equilibrium constant Q38: Iron metal reacts with oxygen gas to Q39: For the following reaction, the equilibrium constant Q40: For the following reaction, the equilibrium constant Q42: The rule or principle that describes the Q43: Write the equilibrium expression for the reaction![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents