The structure of urea is shown below. Fill in any nonbonding valence electrons that are missing from the line-bond structure. 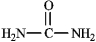
Correct Answer:
Verified
Q7: How many total valence electrons are represented
Q8: Draw all the lone pairs (nonbonding valence
Q8: According to atomic theory:
A) the nucleus is
Q9: Convert the skeletal drawing of the pharmaceutical
Q10: Specify the hybridization of each carbon atom
Q11: Consider the formation of an sp2
Q12: Instructions: Propose a structure for a molecule
Q14: Instructions: Write valid Lewis (electron-dot) structures for
Q14: Covalent bonding
A) involves a transfer of electrons
Q18: In drawing the Lewis structure for an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents