The number of electrons in the n = 4, = 2 subshell in strontium (Z = 38) is ____ the number of electrons in the n =4, =2 subshell in barium (Z = 56) . 
A) times 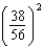
B) times 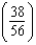
C) equal to
D) times 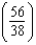
E) times 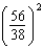
Correct Answer:
Verified
Q30: Rubidium (Z = 37) and potassium (Z
Q32: What angle does the orbital angular
Q33: What is the difference in frequency for
Q34: The ground state configuration of chlorine (Z
Q35: Characteristic x-rays can be produced by bombarding
Q38: The magnitude of the spin angular
Q39: Quantum physics agrees with the classical physics
Q40: Which of the following statements is true?
A)can
Q41: Adam and Eve are contemplating the
Q47: The energy difference between the upper and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents