Given a Galvanic cell: 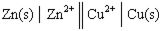 , the right-hand side of this notation represents the:
, the right-hand side of this notation represents the:
A) spontaneous half of the reaction.
B) oxidation 1/2 reaction.
C) anode of the cell.
D) reduction 1/2 reaction.
Correct Answer:
Verified
Q25: How many grams of silver are deposited
Q26: What is the oxidation number of Cl
Q27: Which mechanism is most energy efficient?
A) internal
Q28: What is the E0 value for the
Q29: How long will it take to collect
Q31: What is the E value for the
Q32: An oxidation occurs at the _ of
Q33: The salt bridge between 1/2 reactions maintains
Q34: What is the E value for the
Q35: The SHE is assigned a voltage of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents