What is the E value for the Galvanic cell formed from these two half-reactions at these concentrations?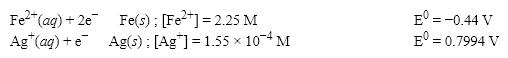
A) 1.00 V
B) 1.12 V
C) 1.24 V
D) 0.36 V
Correct Answer:
Verified
Q29: How long will it take to collect
Q30: Given a Galvanic cell: Q31: What is the E value for the Q32: An oxidation occurs at the _ of Q33: The salt bridge between 1/2 reactions maintains Q35: The SHE is assigned a voltage of Q36: What is the E0 value for the Q37: Single use, non-rechargeable batteries are referred to Q38: What is the E0 value for the Q39: Identify the correct balanced chemical equation for![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents