Multiple Choice
A species containing 16 protons, 17 neutrons, and 15 electrons would be designated as:
A) 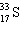
B) 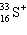
C) 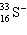
D) 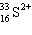
E) none of these
Correct Answer:
Verified
Related Questions
Q31: A Sulfur - 34 isotope has how
Q32: How many protons and neutrons are in
Q33: Which symbol listed below describes a particle
Q34: A particle that contains 8 protons, 9
Q35: A Silicon - 29 isotope has how
Q37: The symbol Q38: The species 28Si2 - has: Q39: How many protons , neutrons and electrons Q40: The symbol Q41: A new element is prepared that has![]()
A) 14![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents