The symbol 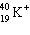 stands for an isotope of potassium containing:
stands for an isotope of potassium containing:
A) 19 electrons, 19 neutrons, 20 protons.
B) 18 electrons, 19 neutrons, 20 protons.
C) 19 electrons, 20 neutrons, 19 protons.
D) 18 electrons, 19 neutrons, 19 protons.
E) 18 electrons, 21 neutrons, 19 protons.
Correct Answer:
Verified
Q32: How many protons and neutrons are in
Q33: Which symbol listed below describes a particle
Q34: A particle that contains 8 protons, 9
Q35: A Silicon - 29 isotope has how
Q36: A species containing 16 protons, 17 neutrons,
Q38: The species 28Si2 - has:
A) 14
Q39: How many protons , neutrons and electrons
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents