What mass of ethane gas (C₂ H₆ ) must burn in excess oxygen to produce 2.00 10 3 kJ of heat, given the thermochemical equation:
2 C₂ H₆(g) + 7 O₂(g) 4 CO₂ (g) + 6 H₂ O ( 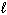 ) D H = - 3.12 10 3 kJ
) D H = - 3.12 10 3 kJ
A) 0.641 g
B) 19.3 g
C) 38.6 g
D) heat is not produced by this reaction
E) none of these
Correct Answer:
Verified
Q1: Given the thermochemical equation:
2 H₂ O₂ (
Q3: Given the equation below, what is D
Q4: Consider the following reaction and its corresponding
Q5: Given the equation below, what is D
Q6: What mass of propane gas (C3 H8
Q7: What mass of nitrogen must burn in
Q8: Liquid hydrogen peroxide is an oxidizing agent
Q9: Given the following reaction and its corresponding
Q10: Energy possessed by a sample of matter
Q11: Which of the following reactions releases heat
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents