Which of the following substances would have a standard enthalpy of formation D H f equal to 0 kJ?
(Pay careful attention to the phase indicators.)
I. H₂ O ( 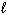 )
)
II. Fe (g)
III. C (s; graphite)
A) I only
B) II only
C) III only
D) I and III
E) II and III
Correct Answer:
Verified
Q52: Given the thermochemical equations and their corresponding
Q53: Which of the following substances is expected
Q54: When a 2.00 g sample of ammonium
Q55: Given the following two equations and their
Q56: Given the following thermochemical equations and their
Q58: Which unit from those listed below would
Q59: Given the equations shown below and their
Q60: In a calorimetry experiment, 0.500 g of
Q61: Given the data below, what is D
Q62: Given the data below, what is D
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents