Given the equations shown below and their corresponding enthalpies:
H₂ O₂ ( 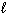 ) H₂ O (
) H₂ O ( 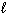 ) + 1 / 2 O₂(g) D H = - 98 kJ H₂ (g) + 1 / 2 O₂(g) H₂ O (
) + 1 / 2 O₂(g) D H = - 98 kJ H₂ (g) + 1 / 2 O₂(g) H₂ O ( 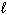 ) D H = - 285 kJ What is D H for the following reaction?
) D H = - 285 kJ What is D H for the following reaction?
H₂ O₂ ( 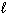 ) H₂ (g) + O₂(g)
) H₂ (g) + O₂(g)
A) - 383 kJ
B) - 187 kJ
C) 383 kJ
D) 187 kJ
E) none of these
Correct Answer:
Verified
Q54: When a 2.00 g sample of ammonium
Q55: Given the following two equations and their
Q56: Given the following thermochemical equations and their
Q57: Which of the following substances would have
Q58: Which unit from those listed below would
Q60: In a calorimetry experiment, 0.500 g of
Q61: Given the data below, what is D
Q62: Given the data below, what is D
Q63: What is the enthalpy of reaction D
Q64: Given the standard heats of formation listed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents