Given the standard heats of formation listed below, what is D H for the following reaction?
C6 H12 O6 (s) + 6 O₂(g) 6 CO₂ (g) + 6 H₂ O ( 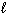 ) D H f C6 H12 O6 (s) = - 1268 kJ/mol D H f CO₂ (g) = - 394 kJ/mol D H f H₂ O (
) D H f C6 H12 O6 (s) = - 1268 kJ/mol D H f CO₂ (g) = - 394 kJ/mol D H f H₂ O ( 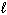 ) = - 286 kJ/mol
) = - 286 kJ/mol
A) - 5332 kJ
B) +588 kJ
C) - 2812 kJ
D) None of these answers are correct (although the problem can be worked with the data given) .
E) Not enough information is given to work the problem.
Correct Answer:
Verified
Q59: Given the equations shown below and their
Q60: In a calorimetry experiment, 0.500 g of
Q61: Given the data below, what is D
Q62: Given the data below, what is D
Q63: What is the enthalpy of reaction D
Q65: Which of the equations below corresponds to
Q66: For which of the equations shown below
Q67: Which of the following defines the heat
Q68: Given the following Thermodynamic data:
D H f
Q69: Given the data below, what is D
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents