For which of the equations shown below does the standard enthalpy of reaction equal the standard molar enthalpy of reaction of the product?
I. 1 / 2 N₂(g) + 3 / 2 H₂ (g) NH₃(g)
II. H₂ (g) + Br₂( 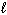 ) 2 HBr (g)
) 2 HBr (g)
III. HCl (g) + NH₃(g) NH ₄Cl (s)
A) I only
B) II only
C) III only
D) I and II
E) I and III
Correct Answer:
Verified
Q61: Given the data below, what is D
Q62: Given the data below, what is D
Q63: What is the enthalpy of reaction D
Q64: Given the standard heats of formation listed
Q65: Which of the equations below corresponds to
Q67: Which of the following defines the heat
Q68: Given the following Thermodynamic data:
D H f
Q69: Given the data below, what is D
Q70: For which of the equations shown below
Q71: What is the enthalpy of reaction D
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents