Lewis structures for three molecules are shown below (assume the connectivity is correct) . Pick the best answer.
I. 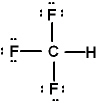
II. 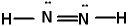
III. 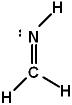
A) I and II are correct, III is not
B) I and III are correct, II is not
C) II and III are correct, I is not
D) all three are correct
E) only I is correct
Correct Answer:
Verified
Q29: Given the three Lewis structures listed below,
Q30: How many electrons (both lone and bond
Q31: How many electrons (both lone and bond
Q32: Given the three Lewis structures below, pick
Q33: Which is the best Lewis structure of
Q35: The molecule N2H4 (H2NNH2 connectivity) has in
Q36: How many electrons (both bond and lone
Q37: How many electrons (both bond and lone
Q38: How many electrons (both lone and bond
Q39: The molecule H2O2 (H - O -
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents