Given the three Lewis structures below, pick the best answer.
I. 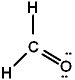
II. 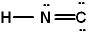
III. 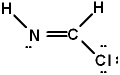
A) All three represent correct Lewis structures.
B) I and II represent correct Lewis structures, III does not
C) I and III represent correct Lewis structures, II does not
D) II and III represent correct Lewis structures, I does not
E) Only I represents a correct Lewis structure.
Correct Answer:
Verified
Q26: The molecule H2S has
A) 2 bonding pairs
Q28: Which Lewis structure below for CO2 obeys
Q29: Given the three Lewis structures listed below,
Q30: How many electrons (both lone and bond
Q31: How many electrons (both lone and bond
Q33: Which is the best Lewis structure of
Q34: Lewis structures for three molecules are shown
Q35: The molecule N2H4 (H2NNH2 connectivity) has in
Q36: How many electrons (both bond and lone
Q37: How many electrons (both bond and lone
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents