Which Lewis structure below for CO2 obeys the octet rule for every atom in the structure and uses the proper number of valence electrons?
A) 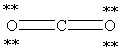
B) 
C) 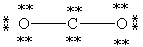
D) 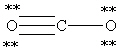
E) 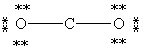
Correct Answer:
Verified
Q23: How many electrons (both lone and bond
Q24: Which Lewis structure below obeys the octet
Q25: The molecule PH3 has:
A) 3 bonding pairs
Q26: The molecule H2S has
A) 2 bonding pairs
Q26: How many electrons (both lone and bond
Q29: Given the three Lewis structures listed below,
Q30: How many electrons (both lone and bond
Q31: How many electrons (both lone and bond
Q32: Given the three Lewis structures below, pick
Q33: Which is the best Lewis structure of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents