Consider the molecule shown below of carbonic acid. Complete its Lewis structure. What are the two approximate bond angles represented by A and B in the figure below?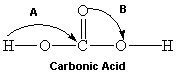
A) A = 180° and B = 90°
B) A = 109° and B = 90°
C) A = 180° and B = 109°
D) A = 109° and B = 120°
E) A = 180° and B = 120°
Correct Answer:
Verified
Q42: What is the H - C -
Q43: Exhibit 10-1 Consider the molecule IF 3
Q44: The H - X - H angle
Q45: In the reaction BH3 + NH3→H3B -
Q46: Below is a structural formula for methyl
Q48: What value listed below best approximates the
Q49: Which molecule listed below has the largest
Q50: Below is a structural formula of ethanol.
Q51: What is the approximate Cl - C
Q52: Exhibit 10-1 Consider the molecule IF 3
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents