Exhibit 10-1 Consider the molecule IF 3 to answer the following question(s) . In theory, there are three possible molecular shapes for the molecule IF 3 as shown below.
I. 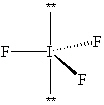
II. 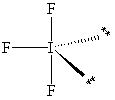
III. 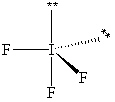
-Refer to Exhibit 10-1. How many significant (90 or less) lone pair/lone pair (LP - LP) interactions are present in each shape?
A) Shape I = 0; Shape II = 0; Shape III = 0
B) Shape I = 0; Shape II = 0; Shape III = 1
C) Shape I = 0; Shape II = 1; Shape III = 0
D) Shape I = 1; Shape II = 0; Shape III = 0
E) Shape I = 1; Shape II = 1; Shape III = 1
Correct Answer:
Verified
Q47: Consider the molecule shown below of carbonic
Q48: What value listed below best approximates the
Q49: Which molecule listed below has the largest
Q50: Below is a structural formula of ethanol.
Q51: What is the approximate Cl - C
Q53: Below is a structural formula of acetic
Q54: What is the O - S -
Q55: What is the approximate H - N
Q56: Pick the one statement listed below that
Q57: Given the three statements below, pick the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents