After carefully noting the orientation in which water molecules surround each solute particle, which choice below best represents the true nature of how Na2SO4 exists in an aqueous solution?
A) 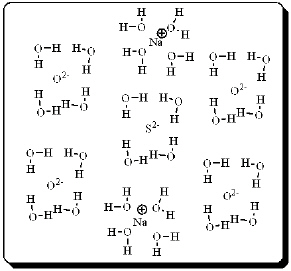
B) 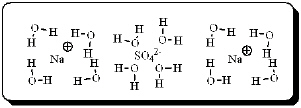
C) 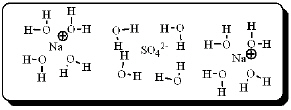
D) 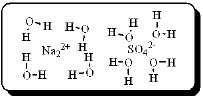
E) 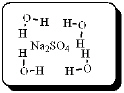
Correct Answer:
Verified
Q95: Which of the following would be expected
Q96: An aqueous solution of Ag2SO4 contains 0.28
Q97: In general, a process that is exothermic
Q98: Which of the following molecular compounds would
Q99: In which solvent would the solubility of
Q101: Which colligative property of solutions listed below
Q102: The partial pressure of carbon dioxide gas
Q103: At 20 ° C the Henry's law
Q104: The solubility of a substance decreases with
Q105: At 10 ° C the Henry's law
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents